Jacobsen Group Research
Catalyst Discovery
Our group has developed several new classes of small-molecule chiral catalysts. The key representative classes are outlined here, and specific examples and applications can be found in the Reaction Gallery.
Asymmetric Catalysis with Chiral Hydrogen-Bond Donors
Efforts spearheaded by our group have opened an entire new sub-field of organocatalysis, in the discovery and development of small-molecule chiral hydrogen-bond donors for asymmetric catalysis. In particular, we have studied dual hydrogen bond donors such as ureas, thioureas, and guanidinium ions in the context of electrophile activation. We elucidated that that these catalytic transformations operate by either of two, fundamentally different modes of electrophile activation. The first involves direct hydrogen bonding with concomitant enhancement of electrophilicity, in a manner analogous to well established pathways in enzymatic catalysis:
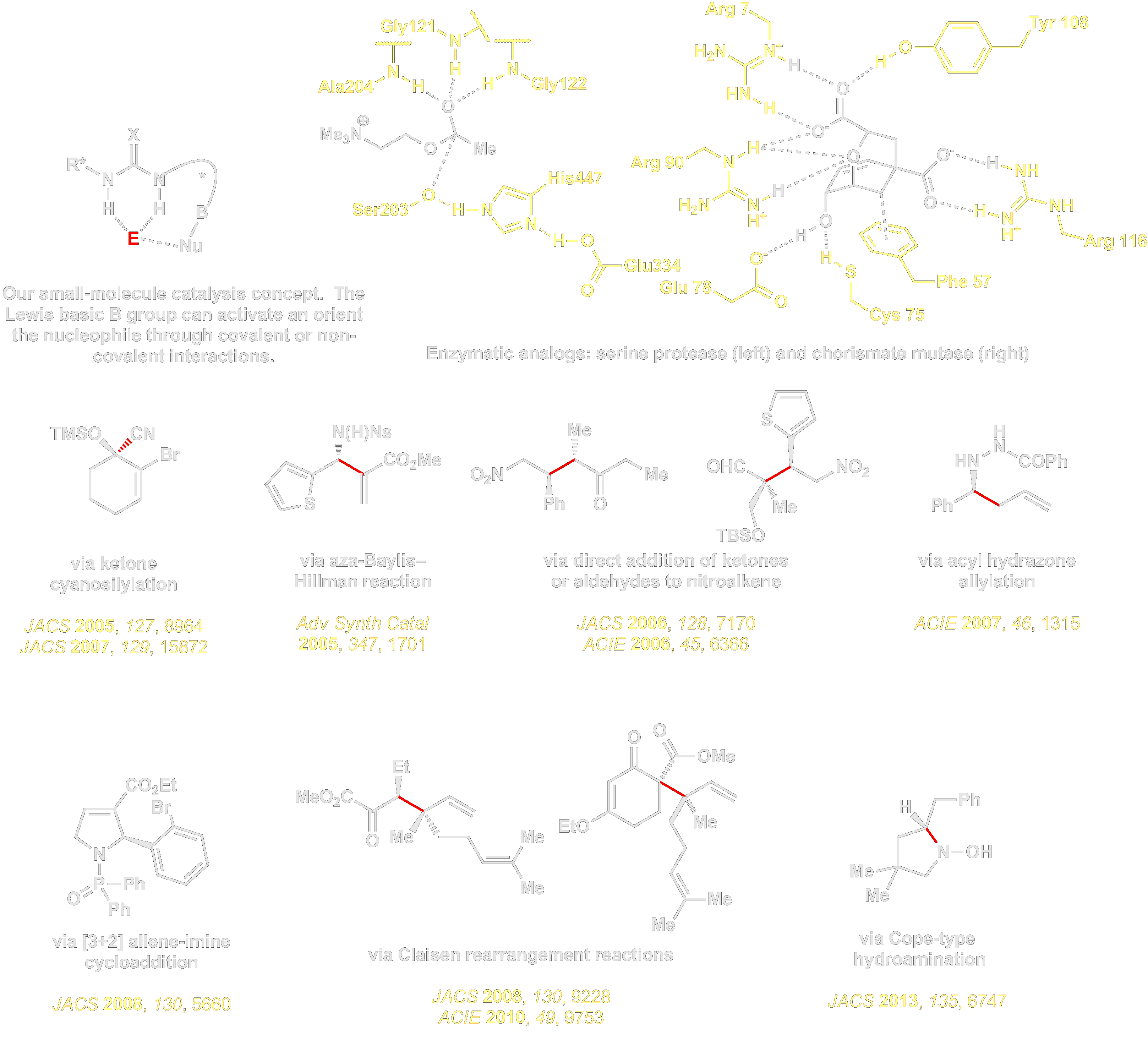
The second involves anion binding catalysis, a concept that provides a general approach to enantioselective catalytic reactions of cationic intermediates such as iminium ions, oxocarbenium ions, halonium ions, unstabilized carbocations, oxidopyrilium ylides, and radical cations. This approach relies on the ability of dual hydrogen bond donors such as ureas and thioureas to abstract or bind weakly basic anions such as halides, sulfonates, and carboxylates. As a result, chiral H-bond donors can generate chiral ion pairs from neutral or ionic electrophiles, and enantioselective reactions can occur if the H-bond donor catalyst remains tightly associated with the cationic intermediates during selectivity-determining events. This approach has served as the foundation for practical methods for a wide range of important processes, including enantioselective catalytic Strecker, Mannich, Povarov, and Pictet–Spengler reactions.
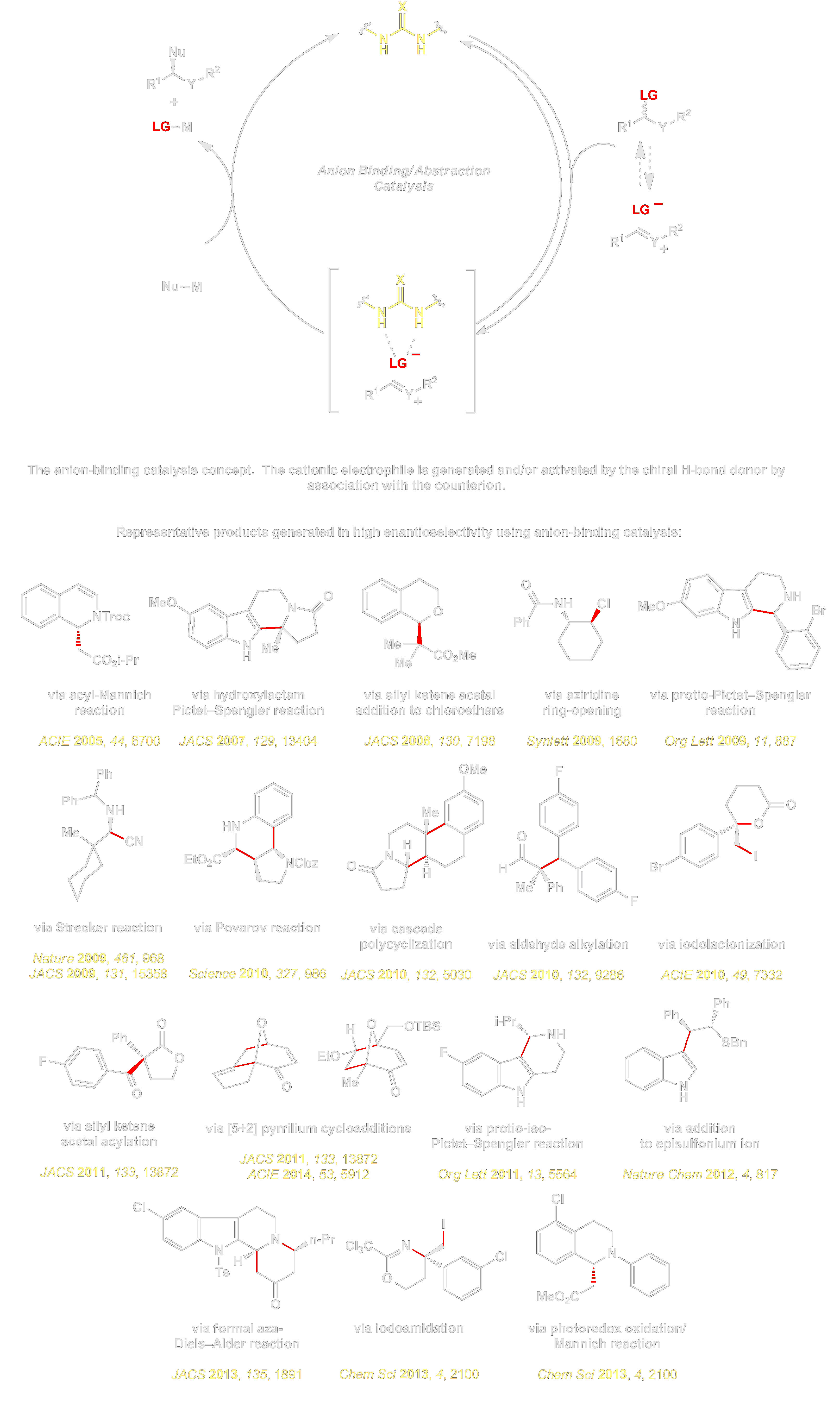
We have found that while simple electrostatic attraction is insufficient to provide the degree of transition state organization necessary for stereocontrol, selectivity can be achieved if additional non-covalent interactions are introduced between the reactive cation and secondary functionality on the catalyst framework. For more on this, you are invited to go to the Mechanism page.
Oxidations
In 1990, our group discovered that manganese complexes of chiral salen ligands catalyze enantioselective epoxidations. Through refinement of the catalyst design and the epoxidation method, we identified the first practical catalysts for the asymmetric epoxidation of simple olefins.
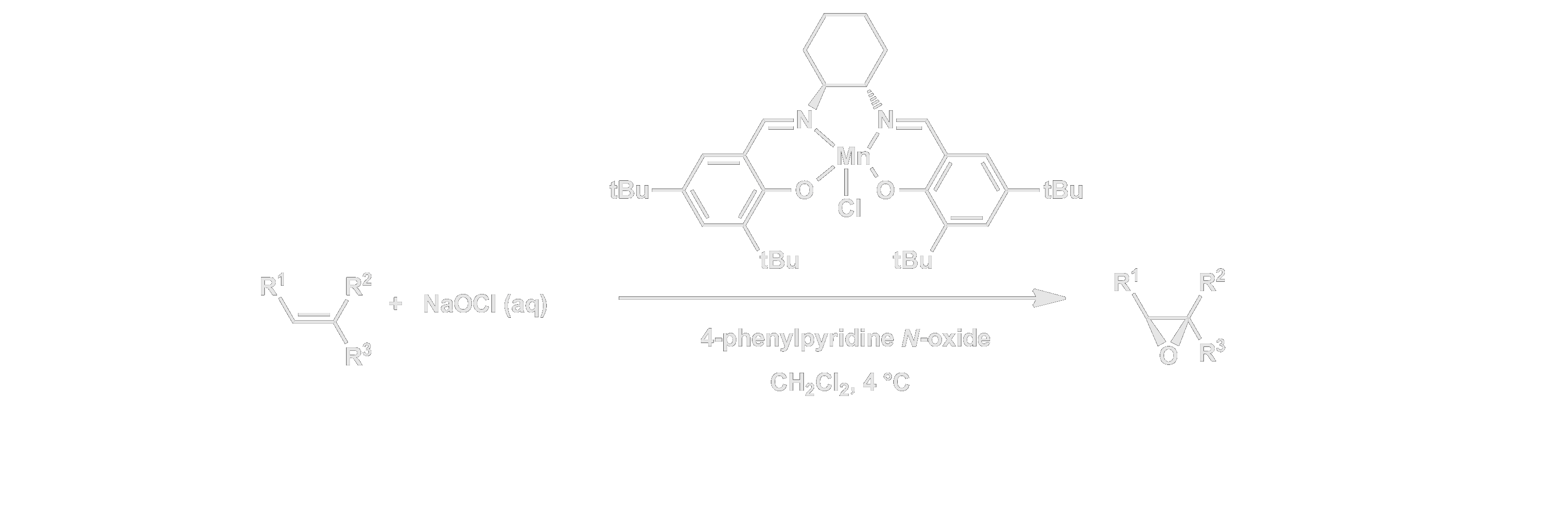
The Mn(salen) catalyst system has been used throughout the world for the synthesis of optically enriched compounds, and it has been developed commercially on a multi-ton scale. Our own group has illustrated the utility of the epoxidation catalysts through their application in key steps of syntheses of several important active compounds, including the antihypertensive agent diltiazem, the medicinally arachidonic acid metabolite leukotriene A4, and the side chain of the anti-cancer drug taxol.
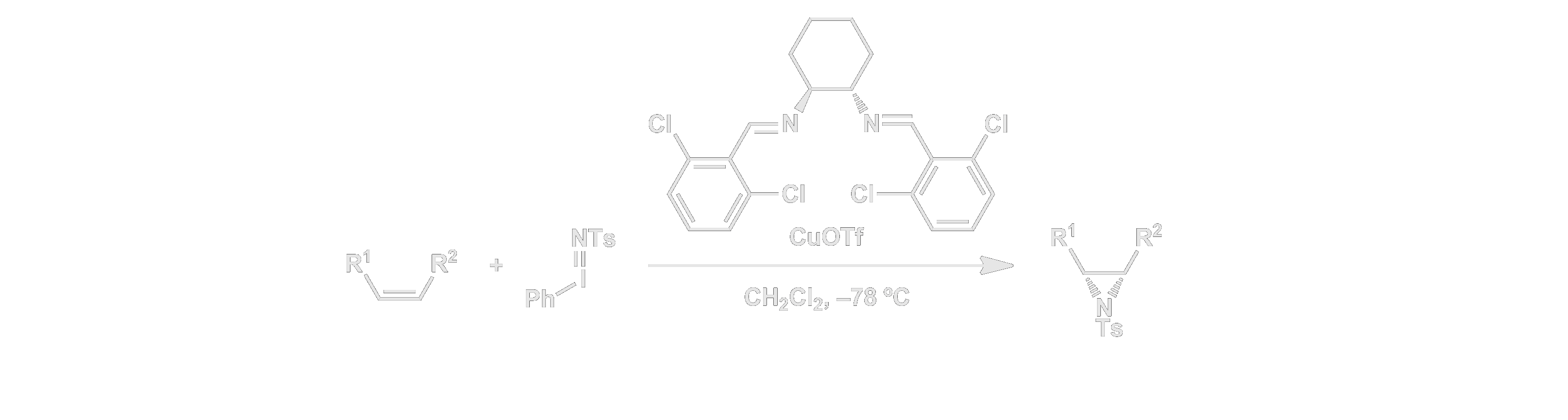
Our group also made significant contributions to the selective transfer of nitrogen-centered oxidants to organic substrates. In particular, we discovered one of the first systems for highly enantioselective catalytic aziridination of alkenes. Again, a most notable feature of these chiral catalysts is their simplicity and accessibility. This work led to useful methods for the synthesis of interesting unnatural amino acid derivatives, and our mechanistic studies provided important insights into the fundamental mechanisms of nitrene transfer.
Epoxide Ring-Opening Reactions
In 1995, our group discovered a highly effective and practical chromium salen-based catalyst system for the desymmetrizations of meso epoxides. The reaction provided a dramatic illustration of efficiency: the catalysts are completely recyclable and can effect ring-opening reactions cleanly, and in the absence of any solvent. As such, the reactions produce no waste whatsoever. We then extended the epoxide ring-opening chemistry to the highly efficient hydrolytic kinetic resolution of terminal epoxides. Under the influence of low loadings of chiral (salen)cobalt complexes, racemic epoxides such as propylene oxide, epichlorohydrin, and butadiene monoepoxide can be resolved by ring-opening with nucleophiles such as water with nearly perfect (>250:1) stereoselectivity. This methodology has allowed access to a wide range of valuable epoxides inexpensively in optically pure form for the first time, and has accordingly had a major impact on organic synthesis at every level. Within just a few years of its discovery, the hydrolytic kinetic resolution (HKR) was applied to the commercial synthesis of several enantiomerically pure epoxides including propylene oxide and epichlorohydrin on multi-ton scale. Laboratory applications of the HKR to synthesis of a wide range of biologically important targets of varying complexity have also emerged, both from within the Jacobsen labs and from several other leading research laboratories.
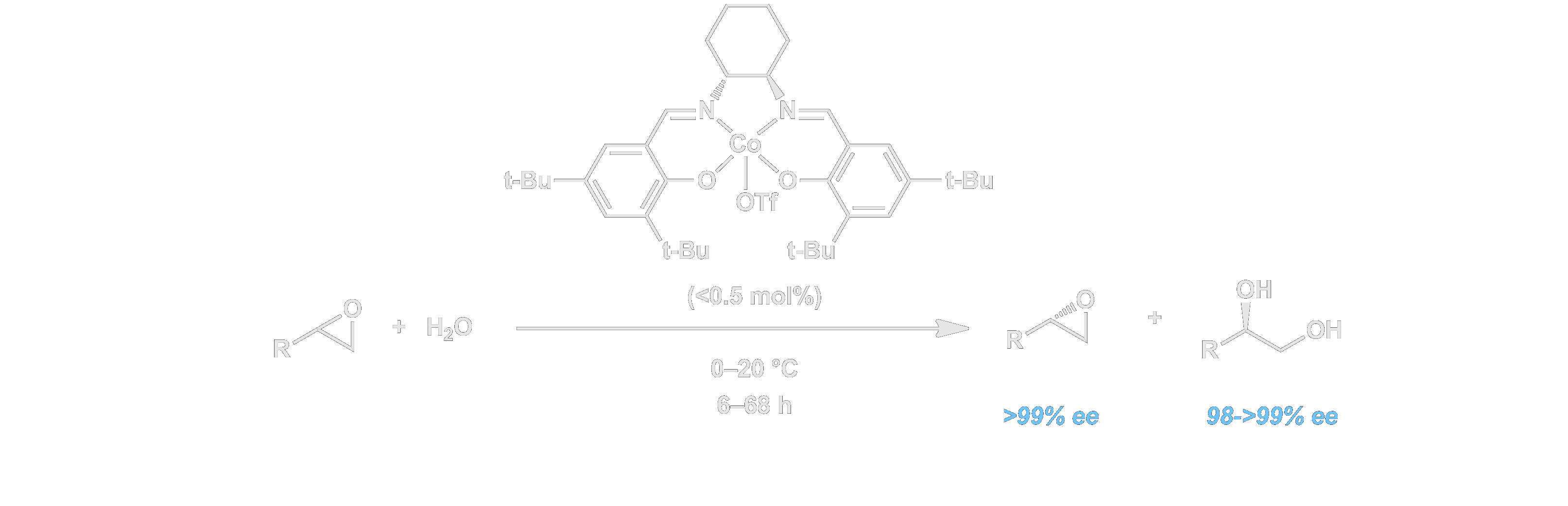
Our mechanistic studies of the HKR led to the elucidation of a cooperative mechanism for the epoxide ring-opening reactions and development of multimeric (salen)Co catalyst with orders-of-magnitude times greater reactivity and broader substrate scope. See the Mechanism section for more on this topic.
Cycloaddition Catalysts
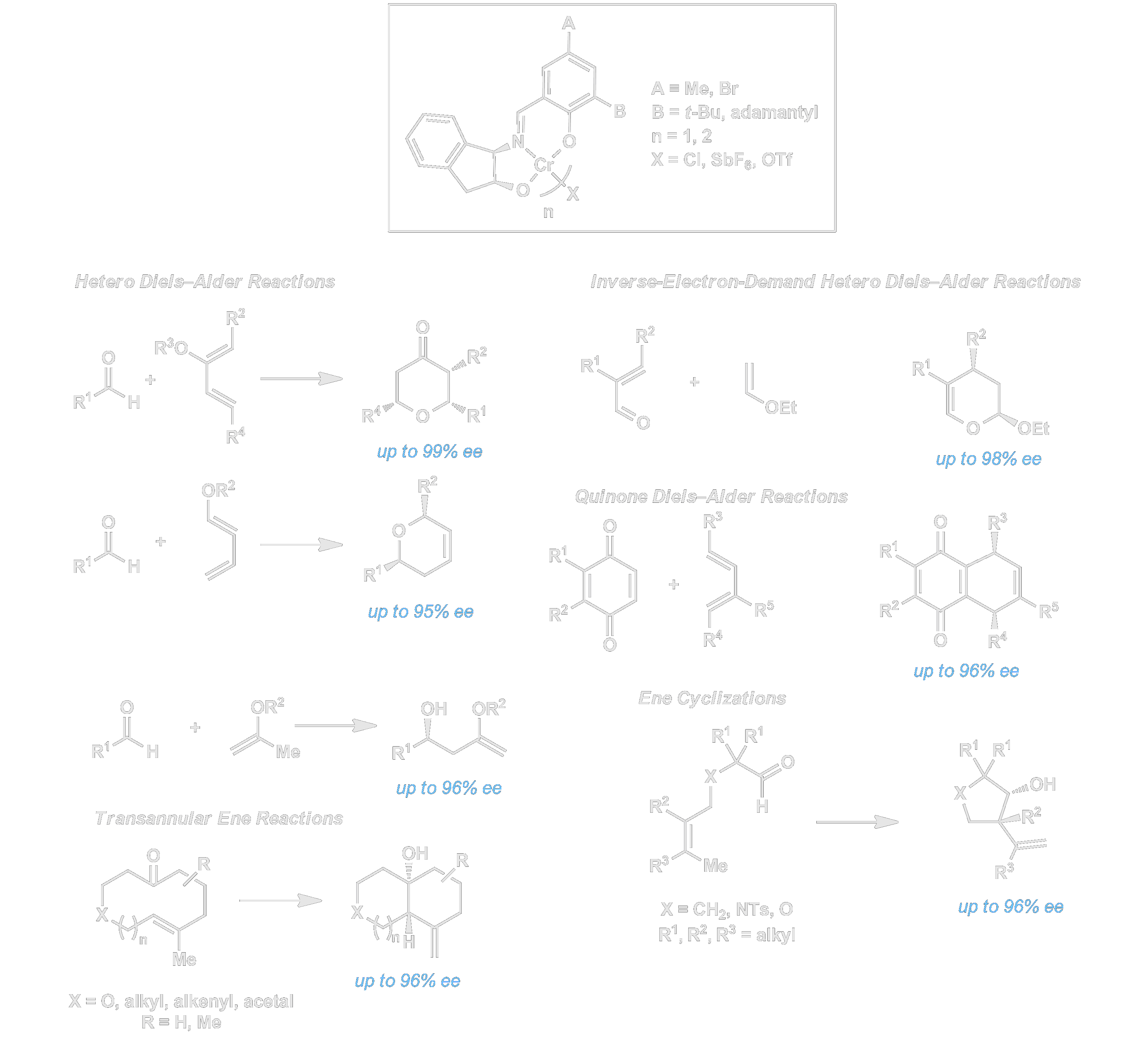
We have developed novel chromium Schiff base complexes for the enantioselective catalytic cycloaddition of simple aldehydes or quinone derivatives with moderately nucleophilic dienes and alkenes. Hetero-Diels–Alder reactions between aldehydes and dienes bearing a single electron-donating substitutent to afford dihydropyranyl products with up to 3 stereogenic centers in nearly perfect diastereoselectivities and high ee’s. The same chromium catalysts promote inverse-electron-demand Diels–Alder reactions with unsaturated aldehydes. Electron-rich alkenes are induced to undergo enantioselective ene reactions with simple aldehydes in intermolecular, intramolecular, and tranannular settings. These reactions have proven broadly useful in natural products synthesis. Perhaps more significant, the chromium catalysts represent a new class of chiral Lewis acids, and have been investigated further in several leading labs throughout the world.
Conjugate Addition Catalysts
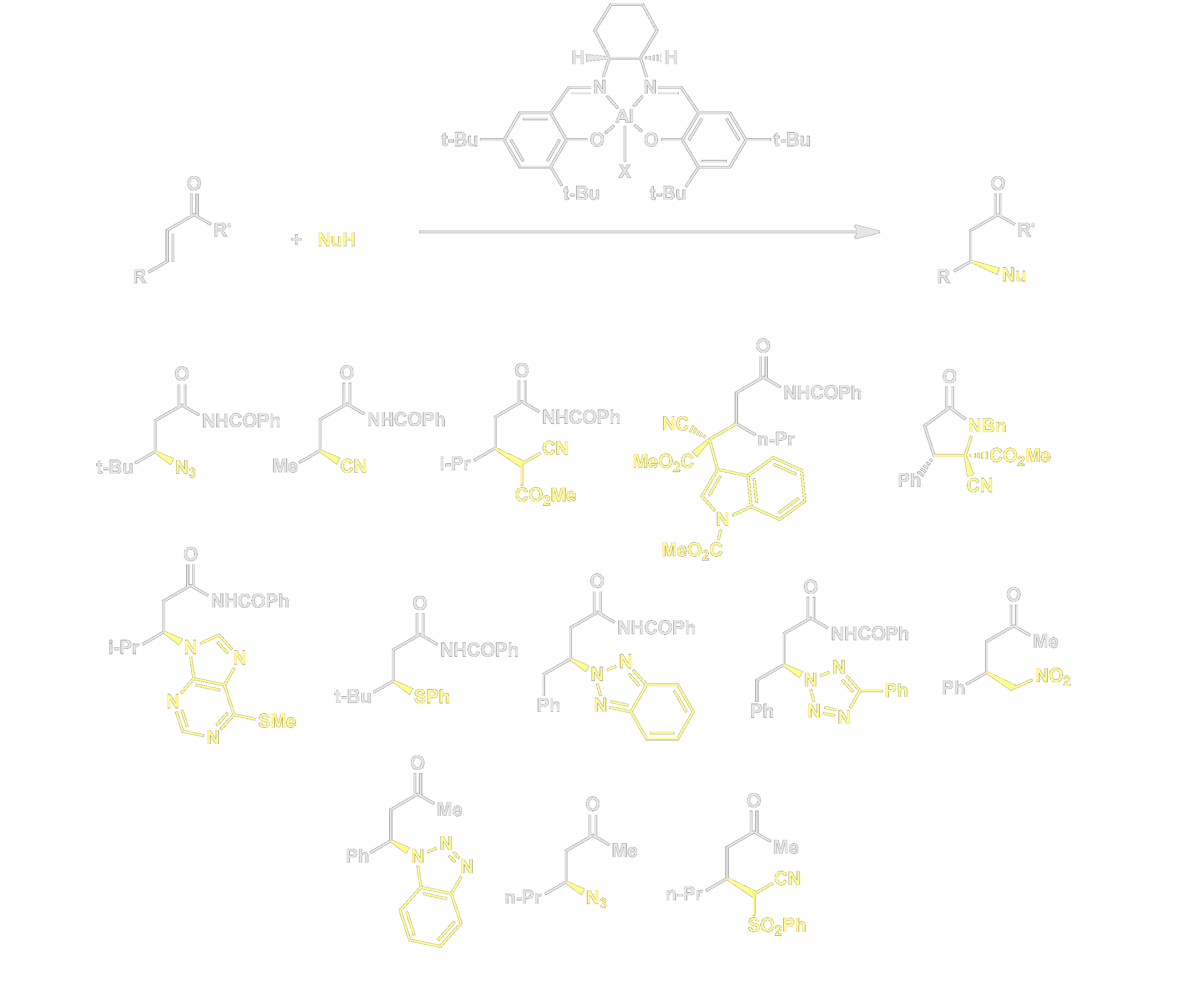
We have discovered that (salen)Al(III) complexes catalyze highly enantioselective conjugate additions of mildly basic nucleophiles to α,β-unsaturated imides and ketones. Remarkable generality is displayed in this chemistry with regard to both nucleophile and electrophile partners, and as a result this methodology has broad synthetic utility. Our kinetic and mechanistic studies on these transformations have revealed that these reactions represent an important example of bimetallic cooperative activation.